1. What Are Mesenchymal Stem Cells (MSCs)?
MSCs (mesenchymal stem or stromal cells) are multipotent adult stem cells found in tissues like bone marrow, adipose, and umbilical cord. They can self-renew and differentiate into bone, cartilage, muscle, and fat cells. Beyond differentiation, MSCs have powerful immunomodulatory and paracrine effects, secreting growth factors, cytokines, and chemokines that orchestrate tissue repair and reduce inflammation. MSCs naturally exist in multiple tissues throughout the human body. Their defining features include their ability to:
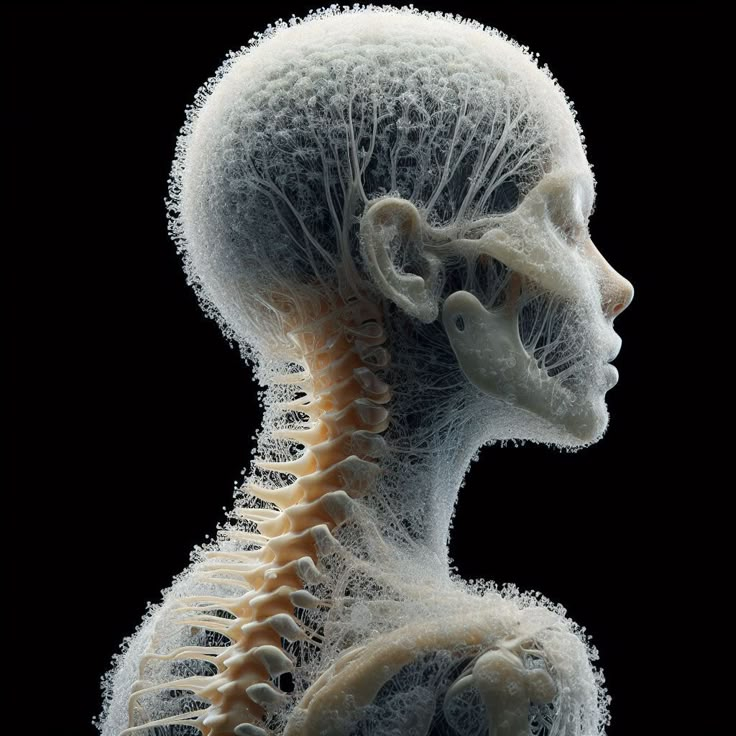
2. Where Do MSCs Come From?
MSCs are most commonly sourced from three main tissues: bone marrow, adipose (fat), and umbilical cord tissue. Each source offers distinct advantages for clinical use.
- Bone Marrow–Derived MSCs:
Traditionally, bone marrow was the gold standard for MSC sourcing. However, collection is invasive, cell yield decreases with patient age, and expansion capacity is limited. - Adipose-Derived MSCs:
Fat tissue is easy to harvest and offers a higher yield of MSCs than bone marrow. Adipose-derived MSCs have robust immunomodulatory properties, but patient age and overall health may impact cell quality. - Umbilical Cord–Derived MSCs:
MSCs from umbilical cord tissue (especially Wharton’s Jelly) are collected non-invasively from healthy, full-term births. These cells are youthful, highly proliferative, and possess strong immunomodulatory abilities. They are considered hypoimmunogenic and suitable for allogeneic (donor-to-patient) use.
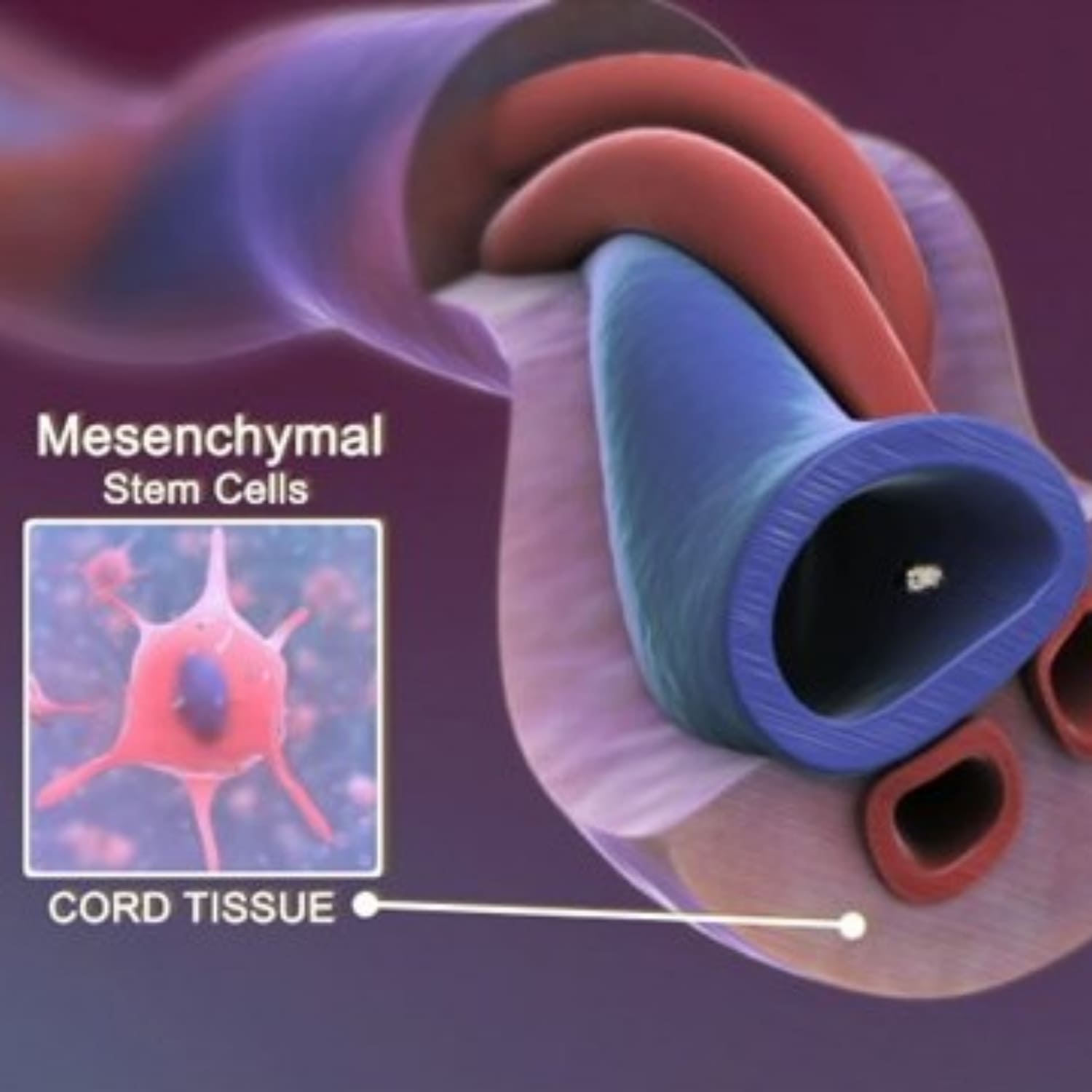
3. Why Umbilical Cord–Derived Stem Cells Are the Optimal Choice
Umbilical cord–derived mesenchymal stem cells (UC-MSCs) are considered the most advantageous source for clinical applications due to their unique biological properties. UC-MSCs are harvested non-invasively from healthy, full-term births, eliminating risks to both mother and child.
These cells are younger and more proliferative than those from adult tissues, allowing for higher yields and superior regenerative potential. UC-MSCs also exhibit lower immunogenicity, which minimizes the risk of rejection and supports safe allogeneic use. Numerous studies have shown that UC-MSCs secrete higher levels of key growth factors and cytokines compared to bone marrow or adipose sources, resulting in stronger anti-inflammatory and immunomodulatory effects.
This combination of safety, potency, and ethical sourcing makes umbilical cord–derived MSCs the preferred choice for advanced regenerative therapies [Jiang et al., 2019; Arutyunyan et al., 2016].
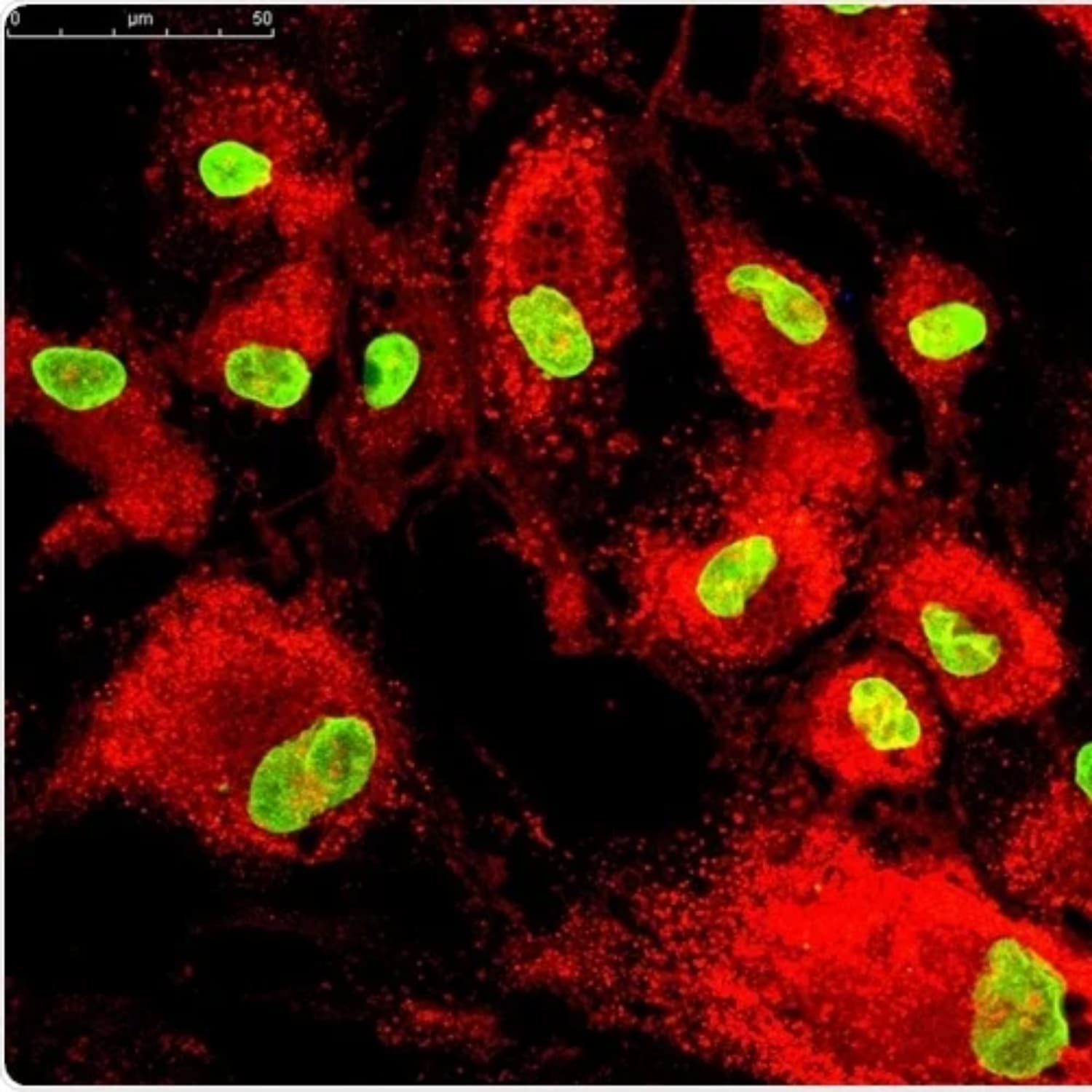
4. Unique Properties of MSCs
MSCs stand out because of their dual action:
- MSCs can become specialized cells such as chondrocytes (cartilage), osteocytes (bone), and adipocytes (fat).
- More important than differentiation, MSCs release anti-inflammatory cytokines, growth factors, and extracellular vesicles (including exosomes) that signal local cells to repair and regenerate tissue.
Key properties include:
- They decrease pro-inflammatory immune responses, supporting healing in autoimmune and degenerative conditions.
- Especially with umbilical cord–derived MSCs, risk of immune rejection is low, making them ideal for donor applications.
- Young sources like umbilical cord provide cells that expand rapidly and secrete abundant healing factors.
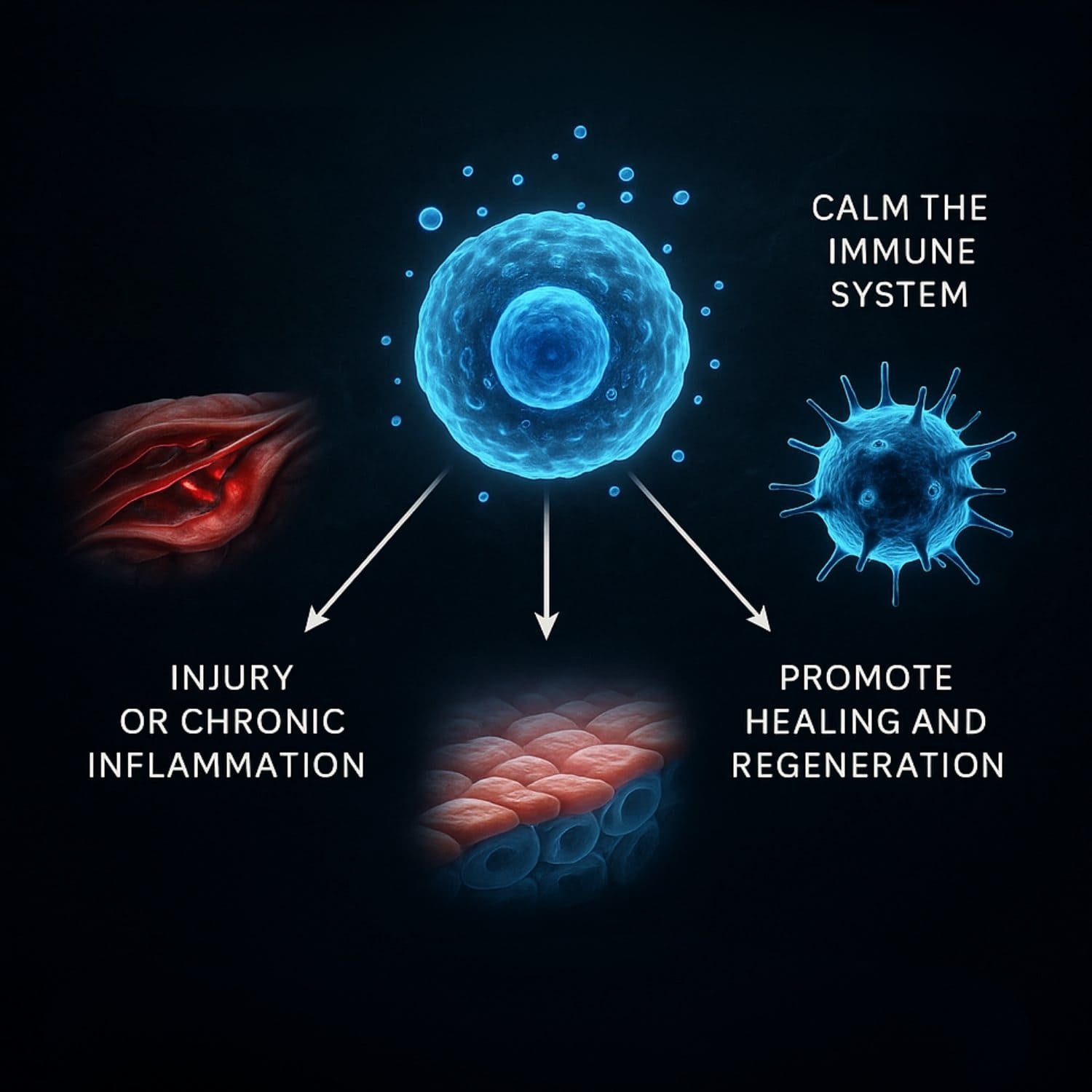
5. Why Are MSCs So Promising in Regenerative Medicine?
- Versatility: Treat a wide range of conditions, from osteoarthritis and ligament injuries to chronic wounds and inflammation.
- Safety: Clinical trials confirm a strong safety profile, especially with allogeneic umbilical cord MSCs.
- Natural Healing: Support the body’s own repair mechanisms, often reducing the need for surgery or chronic medication.
You can explain MSCs to patients with this analogy:
"Think of MSCs as the body’s emergency responders. When there’s injury or chronic inflammation, they go to the affected area, calm the immune system, and send out signals to promote healing and regeneration—often helping your body recover naturally, without surgery."
6. How Do MSCs Work in the Body?
Immunomodulation:
MSCs regulate the immune system by promoting or suppressing inflammation as needed. When the immune system is overactive, as in many autoimmune diseases, MSCs help suppress inflammation. Conversely, when the immune system is underactive, they can enhance immune responses. This ability helps restore immune balance and tissue homeostasis (Bernardo et al., 2012).
Anti-Inflammatory Effects
Chronic, uncontrolled inflammation can cause tissue damage and disease. MSCs reduce harmful inflammation by decreasing pro-inflammatory cytokines such as TNF-α and IFN-γ and increasing anti-inflammatory factors like PGE2 and IL-6 (Gugjoo et al., 2020). These effects are critical in treating autoimmune conditions and supporting tissue repair.
MSC Secretome and Exosome Signaling
The secretome—the collection of molecules released by MSCs—includes cytokines, growth factors, and extracellular vesicles (such as exosomes). These secreted factors regulate cell communication, promote healing, and are increasingly seen as therapeutic agents themselves (Arutyunyan et al., 2016).
Umbilical cord–derived MSCs secrete higher levels of growth factors such as bFGF, NGF, and G-CSF compared to bone marrow or adipose sources, making them particularly potent (Arutyunyan et al., 2016).
Homing Properties
MSCs possess the ability to migrate, or "home," to sites of injury or inflammation within the body. This property allows systemically administered MSCs to exit the bloodstream and localize to damaged tissue, where they contribute to repair (Ullah et al., 2019).
Differentiation Potential
MSCs are multipotent, meaning they can differentiate into various cell types including adipocytes, chondrocytes, osteocytes, and more. This process is controlled by cytokines, growth factors, and extracellular signals and enables MSCs to directly support tissue regeneration (Almalki et al., 2016).
Conclusion
A growing body of research highlights the mechanisms and advantages of mesenchymal stem cells in medicine. MSCs combine self-renewal, immune regulation, anti-inflammatory effects, signaling, and differentiation abilities, making them valuable for a wide range of clinical applications. Umbilical cord–derived MSCs in particular may offer even greater potency, supporting their expanding role in modern regenerative protocols.
Recommended scientific reading:
- Jiang et al., 2019 (PMC6789952)
- Gugjoo et al., 2020 (PMC7404606)
- Almalki et al., 2016 (PMC4756127)
- Arutyunyan et al., 2016 (PubMed)
- Ullah et al., 2019 (PMC6353541)
If you are looking to add MSC Stem cells to your practice, get in touch with the form below.
References
References:
(1) Torres Crigna, A., Daniele, C., Gamez, C., Medina Balbuena, S., Pastene, D. O., Nardozi, D., … Bieback, K. (2018, June 15). Stem/Stromal Cells for Treatment of Kidney Injuries With Focus on Preclinical Models. Frontiers in medicine. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6013716/.
(2) Mazini, L., Rochette, L., Amine, M., & Malka, G. (2019, May 22). Regenerative Capacity of Adipose-Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). International journal of molecular sciences. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6566837/.
(3) Chu, D.-T., Phuong, T. N. T., Tien, N. L. B., Tran, D. K., Thanh, V. V., Quang, T. L., … Kushekhar, K. (2020, January 21). An Update on the Progress of Isolation, Culture, Storage, and Clinical Application of Human Bone Marrow Mesenchymal Stem/Stromal Cells. International journal of molecular sciences. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7037097/.
(4) Jin, H. J., Bae, Y. K., Kim, M., Kwon, S.-J., Jeon, H. B., Choi, S. J., Kim, S. W., Yang, Y. S., Oh, W., & Chang, J. W. (2013, September 3). Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. International journal of molecular sciences. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3794764/.
(5) Liau, L. L., Looi, Q. H., Chia, W. C., Subramaniam, T., Ng, M. H., & Law, J. X. (2020, September 22). Treatment of spinal cord injury with mesenchymal stem cells. Cell & bioscience. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7510077/.
(6) Jiang, W., & Xu, J. (2020, January). Immune modulation by mesenchymal stem cells. Cell proliferation. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6985662/.
(7) Bernardo, M. E., & Fibbe, W. E. (2013). Mesenchymal Stromal Cells: Sensors and Switchers of Inflammation. Cell Stem Cell, 13(4), 392–402. https://doi.org/10.1016/j.stem.2013.09.006
(8) Ryu, J.-S., Jeong, E.-J., Kim, J.-Y., Park, S. J., Ju, W. S., Kim, C.-H., Kim, J.-S., & Choo, Y.-K. (2020, November 7). Application of Mesenchymal Stem Cells in Inflammatory and Fibrotic Diseases. International journal of molecular sciences. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7664655/.
(9) Gugjoo, M. B., Hussain, S., Amarpal, Shah, R. A., & Dhama, K. (2020). Mesenchymal Stem Cell-Mediated Immuno-Modulatory and Anti- Inflammatory Mechanisms in Immune and Allergic Disorders. Recent patents on inflammation & allergy drug discovery. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7509741/.
(10) Stastna, M., & Van Eyk, J. E. (2012, February 1). Investigating the secretome: lessons about the cells that comprise the heart. Circulation. Cardiovascular genetics. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3282018/.
(11) Ullah, M., Liu, D. D., & Thakor, A. S. (2019, May 31). Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6529790/.
(12) Almalki, S. G., & Agrawal, D. K. (2016). Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation; research in biological diversity. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5010472/.
(13) Grafe, I., Alexander, S., Peterson, J. R., Snider, T. N., Levi, B., Lee, B., & Mishina, Y. (2018, May 1). TGF-β Family Signaling in Mesenchymal Differentiation. Cold Spring Harbor perspectives in biology. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5932590/.
(14) Walker, J. T., Keating, A., & Davies, J. E. (2020, May 28). Stem Cells: Umbilical Cord/Wharton’s Jelly Derived. Cell Engineering and Regeneration. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7992171/.
(15) Arutyunyan, I., Elchaninov, A., Makarov, A., & Fatkhudinov, T. (2016). Umbilical Cord as Prospective Source for Mesenchymal Stem Cell-Based Therapy. Stem cells international. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5019943/.