MSC Stem Cell & Exosome Resource Center for Physicians
Access everything you need to confidently offer mesenchymal stem cell and exosome therapies in your clinic.
From scientific research and clinical protocols to legal compliance guides and patient education, this resource hub empowers Florida physicians to lead in safe, effective regenerative care.
Stay informed. Stay compliant. Transform patient outcomes with BioRegen.
FAQs
Our stem cell lines and exosomes are sourced from FDA Type II DMF-registered tissue banks and follow 361 HCT/P regulatory compliance for research and clinical investigational use.
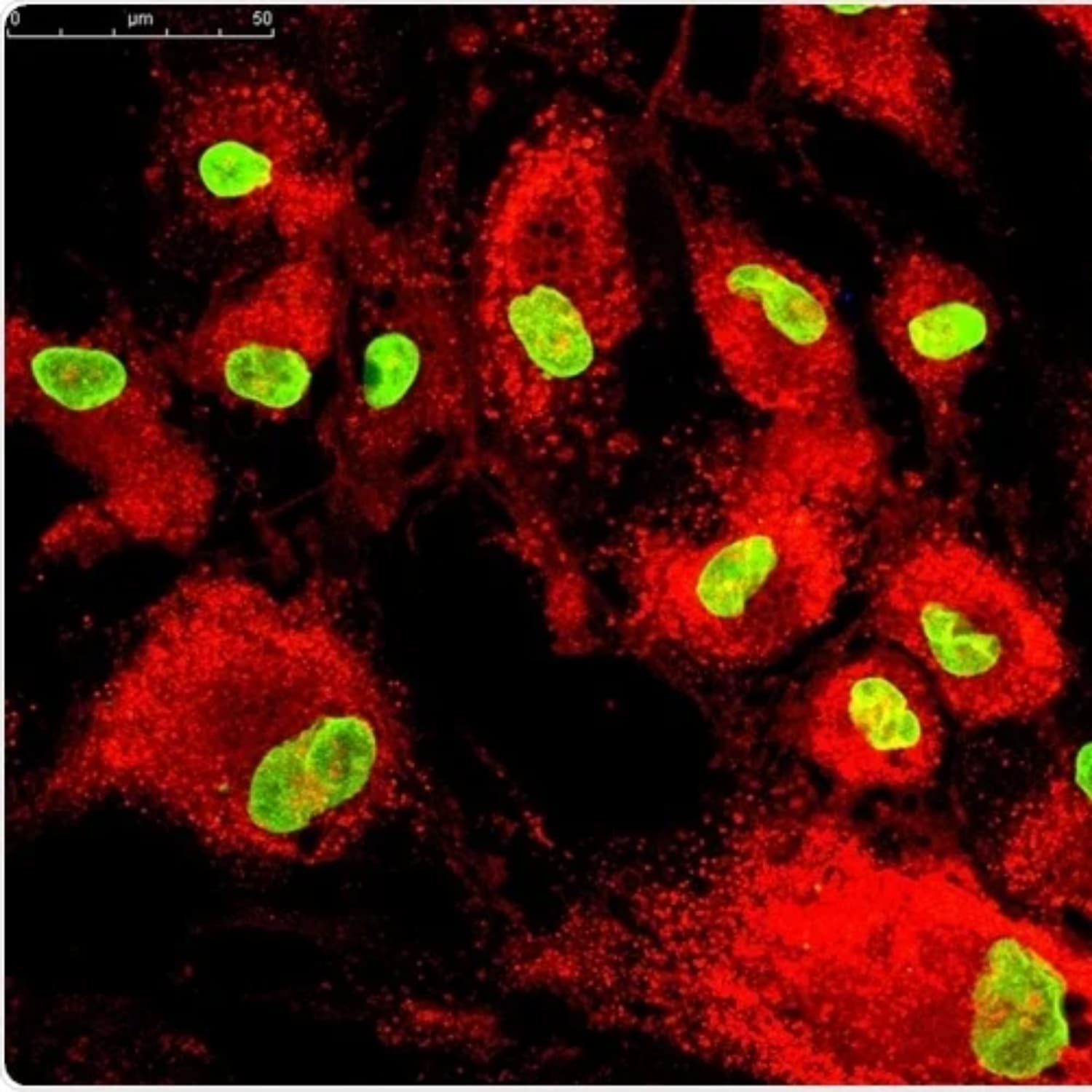
3D culturing mimics the human body’s natural environment, enabling up to 20x more secretion of healing factors like exosomes, cytokines, and growth factors.
All biologics are manufactured to injectable quality standards, though they are labeled for topical and research use only unless used under appropriate physician discretion or applicable law (e.g., Right to Try).
Doctors and clinics use our solutions for joint regeneration, aesthetic recovery, post-laser treatments, hair restoration, autoimmune support, and neuro-repair protocols—often in stacked regimens.
Resources
The ultimate resource hub for Florida physicians on mesenchymal stem cells (MSCs) and exosomes. Access expert guides, clinical protocols, legal compliance checklists, and patient education materials to confidently integrate regenerative therapies into your medical practice. Stay ahead in stem cell science, safety, and regulations with BioRegen’s comprehensive library for doctors.